The first FDA conditionally approved innovative solution for the management of clinical signs associated with acute onset of pancreatitis in dogs

Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-567. It is a violation of Federal law to use this product other than as directed in the labeling.
Introducing
The first FDA conditionally approved innovative solution for the management of clinical signs associated with acute onset of pancreatitis in dogs

Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-567. It is a violation of Federal law to use this product other than as directed in the labeling.
1/3
Pancreatitis is an inflammatory disease.
Neutrophilic inflammation is a hallmark of ACP.1 LFA-1 (leukocyte function-associated antigen-1) activation is a critical component of pancreatic inflammation.
2/3
Inflammation control with a unique mechanism of action.
Fuzapladib sodium for injection (PANOQUELL®-CA1) is an LFA-1 activation inhibitor.
3/3
Anti-inflammatory effects through LFA-1 inhibition:
May limit pancreatic lesion expansion.
May help prevent complications such as multi-organ failure.
Introducing the first FDA conditionally approved innovative solution for the management of clinical signs associated with acute onset of pancreatitis in dogs

Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-567. It is a violation of Federal law to use this product other than as directed in the labeling.
Pancreatitis is an inflammatory disease.
Neutrophilic inflammation is a hallmark of ACP.1 LFA-1 (leukocyte function-associated antigen-1) activation is a critical component of pancreatic inflammation.
Inflammation control with a unique mechanism of action.
Fuzapladib sodium for injection (PANOQUELL®-CA1) is an LFA-1 activation inhibitor.
Anti-inflammatory effects through LFA-1 inhibition:
May limit pancreatic lesion expansion.
May help prevent complications such as multi-organ failure.
Changing the standard of care for canine pancreatitis.
Based on the data submitted by the sponsor for the conditional approval of PANOQUELL®-CA1, FDA determined that the drug is safe and has a reasonable expectation of effectiveness when used according to the labeling. The effectiveness of fuzapladib sodium was demonstrated in a well-controlled pilot field study. Dogs treated with fuzapladib sodium had a statistically significant reduction in MCAI scores compared to control.2
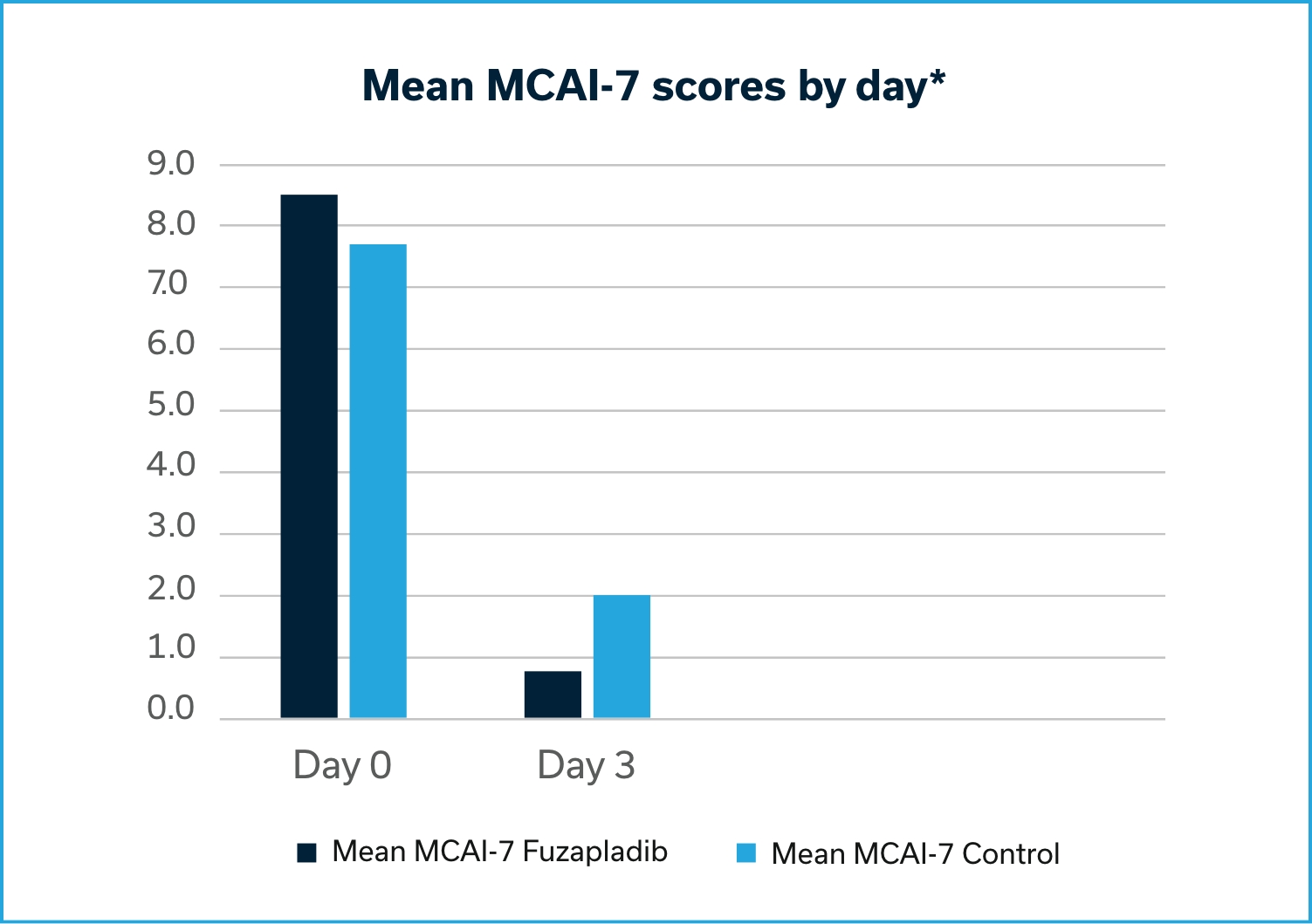
A Modified Canine Activity Index (MCAI) was used to evaluate and score seven clinical signs relevant in dogs with acute pancreatitis: Activity, Appetite (voluntary food intake), Vomiting, Cranial abdominal pain, Dehydration, Stool consistency, and Blood in the stool. The primary effectiveness variable was the change in the group mean total MCAI score from Day 0 (pre-treatment) to Day 3, as assessed by the Investigator. Day 0 mean MCAI scores for the fuzapladib sodium and vehicle control groups were 8.53 and 7.68, respectively. The changes in the mean total MCAI scores from Day 0 to 3 for the fuzapladib sodium and vehicle control groups were -7.7 and -5.7, respectively.
*Dogs treated with fuzapladib sodium had a statistically significant reduction in MCAI scores compared to control (p=0.0193).
Based on the data submitted by the sponsor for the conditional approval of PANOQUELL®-CA1, FDA determined that the drug is safe and has a reasonable expectation of effectiveness when used according to the labeling. The effectiveness of fuzapladib sodium was demonstrated in a well-controlled pilot field study. Dogs treated with fuzapladib sodium had a statistically significant reduction in MCAI scores compared to control.2
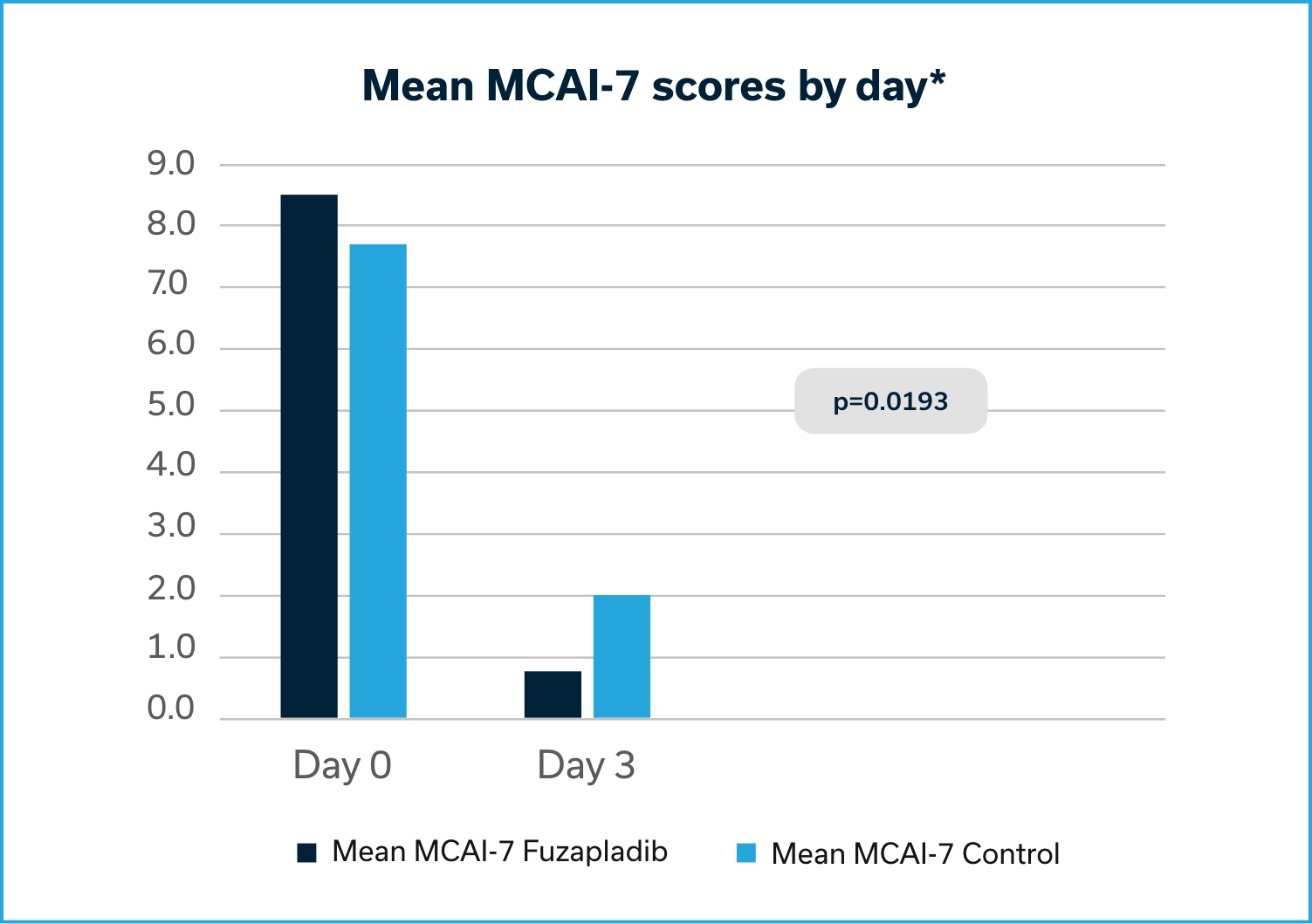
A Modified Canine Activity Index (MCAI) was used to evaluate and score seven clinical signs relevant in dogs with acute pancreatitis: Activity, Appetite (voluntary food intake), Vomiting, Cranial abdominal pain, Dehydration, Stool consistency, and Blood in the stool. The primary effectiveness variable was the change in the group mean total MCAI score from Day 0 (pre-treatment) to Day 3, as assessed by the Investigator. Day 0 mean MCAI scores for the fuzapladib sodium and vehicle control groups were 8.53 and 7.68, respectively. The changes in the mean total MCAI scores from Day 0 to 3 for the fuzapladib sodium and vehicle control groups were -7.7 and -5.7, respectively.
*Dogs treated with fuzapladib sodium had a statistically significant reduction in MCAI scores compared to control (p=0.0193).
In the pilot field effectiveness study, PANOQUELL®-CA1 was administered safely with other supportive care treatments including anti-emetics and pain control. The most common adverse reactions included anorexia, digestive tract disorders, respiratory tract disorders and hepatopathy and jaundice.
In a good laboratory practice, pivotal target animal safety study, the administration of PANOQUELL®-CA1 as an IV injection once daily for nine days at doses of 0, 0.4, 1.2, and 2 mg/kg fuzapladib sodium did not produce systemic toxicity and had an acceptable margin of safety. The administration of PANOQUELL®-CA1 resulted in swelling and bruising at the injection site, with associated gross pathology and histopathological findings, hypertension, and mild thrombocytopenia. This nine-day safety study supports the safe use of PANOQUELL®-CA1 when administered IV to dogs, according to the label.2

PANOQUELL®-CA1 is cost effective. The multi-use vial treats multiple patients, thus reducing waste.
Contact your Ceva representative or request additional information below to find out more.

Questions?
Click below to request more information!
For product support call 1-800-999-0297.
1Cridge H, Lim SY, Algül H, Steiner JM. New insights into the etiology, risk factors, and pathogenesis of pancreatitis in dogs: Potential impacts on clinical practice. J Vet Intern Med. 2022 May;36(3):847-864. doi: 10.1111/
2https://animaldrugsatfda.fda.gov/adafda/



